CREDIT: GAO LIU/BERKELEY LAB. COURTESY OF NATURE ENERGY.
Scientists at Lawrence Berkeley National Laboratory developed a conductive polymer coating – called HOS-PFM – that could enable longer lasting, more powerful lithium-ion batteries for electric vehicles.
“The advance opens up a new approach to developing EV batteries more affordable and easy to manufacture,” says Gao Liu, a senior scientist in Berkeley Lab’s Energy Technologies Area.
The HOS-PFM coating conducts both electrons and ions at the same time. This ensures battery stability and high charge/discharge rates while enhancing battery life. The coating also shows promise as a battery adhesive that could extend the lifetime of a lithium-ion battery from an average of 10 years to about 15 years.
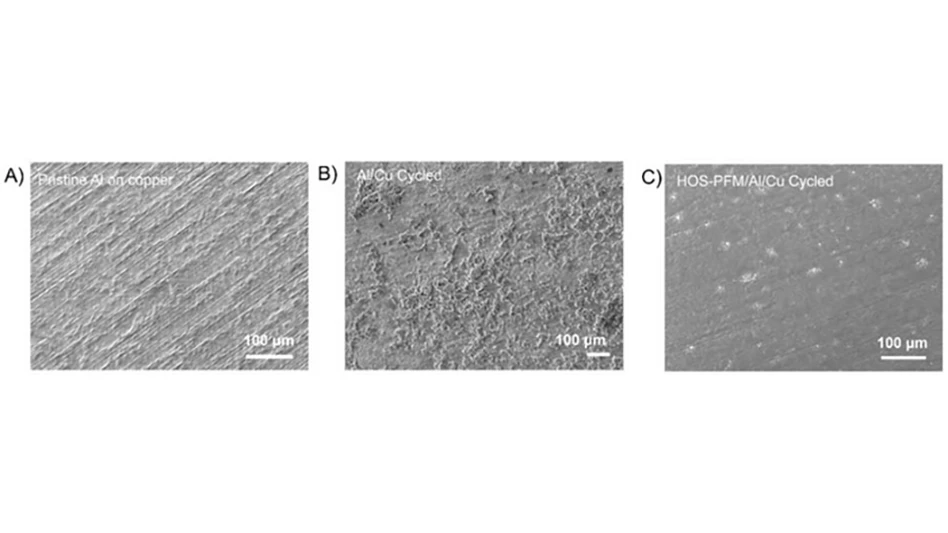
To demonstrate HOS-PFM’s superior conductive and adhesive properties, Liu and his team coated aluminum and silicon electrodes with HOS-PFM, and tested their performance in a lithium-ion battery setup.
Silicon and aluminum are promising electrode materials for lithium-ion batteries because of their potentially high energy storage capacity and lightweight profiles. But these cheap and abundant materials quickly wear down after multiple charge/discharge cycles.
During experiments at the Advanced Light Source and the Molecular Foundry, the researchers demonstrated that the HOS-PFM coating significantly prevents silicon- and aluminum-based electrodes from degrading during battery cycling while delivering high battery capacity over 300 cycles, a performance rate that’s on par with today’s state-of-the-art electrodes.
The results are impressive because silicon-based lithium-ion cells typically last for a limited number of charge/discharge cycles and calendar life.
The HOS-PFM coating could allow the use of electrodes containing as much as 80% silicon. Such high silicon content could increase the energy density of lithium-ion batteries by at least 30%, Liu said. And because silicon is cheaper than graphite, the standard material for electrodes today, cheaper batteries could significantly increase the availability of entry-level electric vehicles, he added.
The team next plans to work with companies to scale up HOS-PFM for mass manufacturing.
The Advanced Light Source and Molecular Foundry are DOE Office of Science user facilities at Berkeley Lab.
The research was supported by DOE Vehicle Technologies Office.
Latest from EV Design & Manufacturing
- Powering homes with EV batteries could cut emissions, save thousands of dollars
- Meviy introduces stainless steel passivation option for CNC, sheet metal parts
- December Lunch + Learn webinar with Fagor Automation
- December Lunch + Learn webinar with LANG Technik + Metalcraft Automation Group
- EVIO makes public debut with hybrid-electric aircraft
- Redesigned pilot step drill triples performance
- Green Energy Origin expands battery electrolyte manufacturing in North America, Europe
- What’s next for the design and manufacturing industry in 2026?





